When novel materials may be released into the environment, it is necessary to ensure they will not have a negative impact on the organisms and processes that make up that environment.
Algae as primary producers are an important component of the ecosystem and representative species are used to test for adverse effects of materials. At Heriot-Watt University, researchers are investigating the ecotoxicology of nanoparticles and nanomaterials. These are solid materials, for the most part insoluble in water, that are manufactured as very small particles, less than 100nanometres (1/10,00 of a millimetre) in at least 1 dimension. They have properties which are very different from ‘normal’ sized particles and have been incorporated in consumer products for several years, e.g. in sunscreens, clothing (antibacterial socks), self-cleaning window treatments and medical applications.
At present our knowledge of what happens to the particles when the products are used (e.g. socks are washed) is limited, and research groups around the world are attempting to fill this gap. Possible adverse effects on organisms are investigated by exposing test species to a range of concentrations of the nanomaterials. The organisms generally recognised as suitable test species include fish, invertebrates (aquatic worms, Lumbriculus, and the water flea Daphnia) and freshwater or marine algae.
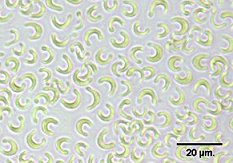
The unicellular green alga Pseudokirchneriella subcapitata (formerly known as Selenastrum capricornutum, or briefly as Raphidocelis subcapitata) has been a freshwater species of choice for many years. Testing for toxic effects is carried out by comparing the growth of cultures exposed to the toxicant with the growth of control (unexposed) cultures.
Growth is taken to be an increase in biomass of the culture. There are different techniques for assessing this. The most basic is to filter the culture and weigh the filter, but this requires large culture volumes and is inefficient, and is not applicable when the toxicant is particulate (even if the nanoparticles are so small they ought to pass through the filter).
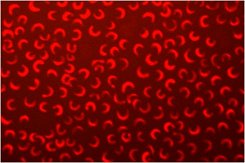
Usually a proxy for biomass is used, such as cell number or optical density. Cell number can be assessed in a counting chamber such as a haemacytometer (designed for counting blood cells using a microscope), or using a more complex piece of equipment called a flow cytometer, which uses a laser beam to count cells as they flow past. The cell density may also be assessed using a spectrophotometer, which measures the effect of the cells in blocking the passage of a light beam through the culture. This is a very rapid and simple method but suffers from interference by some particles.
Fluorescence is a similar but more sensitive technique which relies on the presence of chlorophyll within the cells, but has the same potential for interference from particles. However, it is relatively simple to extract the chlorophyll from a sample of the culture using a solvent (acetone) and allow the cell remnants and particles to settle out before using the fluorometer to assess the chlorophyll concentration. This procedure is being used to compare the toxicities of different types of particle (different chemistry, particle size, coating), under different conditions.